Volcanic gases and hot springs
火山研究解説集:薩摩硫黄島 (産総研・地質調査総合センター作成)
| Contents: Satsuma-iojima |
|---|
|
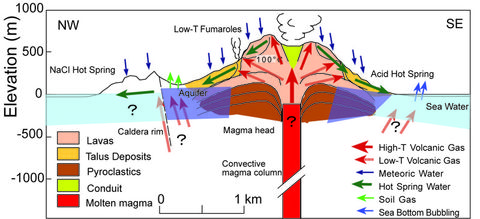
- Cross section showing differentiation process of volcanic gas and hot spring
Introduction
Iodake of Iojima actively emits volcanic plume (volcanic gas) for more than several hundreds years.
Volcanic gases released from the magma beneath the Iodake are the cause of various phenomena such as fumaroles, volcanic plume, hot springs on the beach, soil gas, discolored sea water, gas bubbles on the sea floor, formation of silicified rocks by alteration of existing rocks at the summit etc.
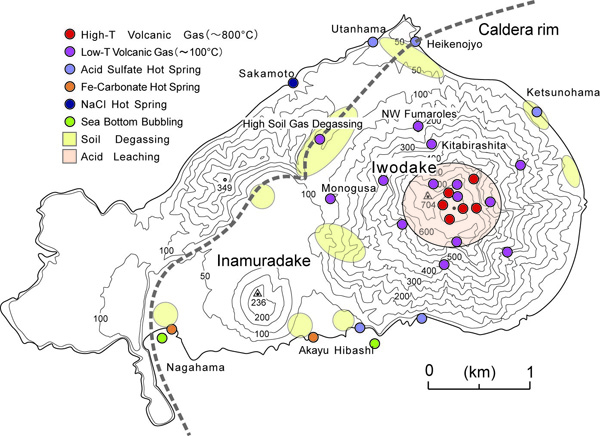
- Distribution of volcanic gas, hot spring, and soil gas
High temperature volcanic gas, low temperature volcanic gas, soil gas and hot springs are concentrically distributed with the summit of Iodake at their center (See Distribution map of gases and hot springs).
The distribution of gases and hot springs is controlled by underground movement of volcanic gases and groundwater as well as temperature distribution as shown in the cross section showing differentiation process of volcanic gas and hot spring.
Pit crater within the summit crater
- Floor of Summit crater bottom in 2000
Within the summit crater of Iodake is a pit crater 200 m in diameter which discharges volcanic gases. The pit crater was formed accompanied by an eruption of large volume of ash in 1990s.
The photo above shows the floor of summit crater in October 2000. The diameter of the pit crater at that time was about 50 m and continuously discharging ash.
High temperature volcanic gas
High temperature fumaroles with temperatures at 800 to 900℃are distributed around the summit crater of Iodake (See Distribution map of volcanic gas and hot springs).
Near the Ohachi fumarolic zone shown in the photo, an alteration zone in whitish gray is developed around high temperature fumaroles surrounded by sublimation deposits rich in heavy metals. Especially striking is bright molybdenum blue color deposits.
At high temperature fumaroles red hot colors inside of the fumaroles can be seen even in the daytime (Photo of the highest temperature fumarole at Ohachi-Oku). The temperature registered here is just slightly lower than that of the magma suggesting the gas was released from the magma existing close to the ground surface. Because of this, high temperature volcanic gas has been studied to obtain information on the magma.
Volcanic gas from the summit
Fumaroles of various temperatures spanning from 100 to 800℃ are distributed near the summit of Iodake (See Distribution map of volcanic gas and hot springs).
Those gases of summit area show similar compositions to the high temperature volcanic gas and interpreted as cooled equivalents of the high temperature gas.
Around the group of low temperature fumaroles within the summit crater, sulfur precipitated from the volcanic gas forms chimneys.
Low temperature volcanic gas at foothill areas
Low temperature fumaroles at around 100℃ are distributed along valleys on the flanks of Iodake (See Distribution map of volcanic gas and hot springs).
Low temperature volcanic gas at the foot of the volcano is derived from boiling acidic water, which is produced from mixing of cooled gas and groundwater (originated from rain water). It can be compared to the steam from boiling kettle, and its temperature is close to 100℃. As water-soluble acid gas components such as sulfur dioxide and hydrogen chloride are retained in the condensed acidic hot water, low temperature volcanic gas lacks in those components.
Hydrothermally altered rocks
Near the summit is distributed silicastone (See Distribution map of volcanic gas and hot springs) (Photo: Otanibira silicastone quarry. Refer also for the map showing location of Otanibira and distribution of alteration zone).
Silicified rocks was formed by reaction between acid hot water condensed from volcanic gas and volcanic rocks, resulting in leaching all the components other than silica (SiO2). The silicastone was once used to be mined for house-building material.
Acidic sulfate hot springs
As Higashi Hot Spring shown in the left photo, strongly acidic hot springs (sulfate hot springs) (See Distribution map of volcanic gas and hot springs) are distributed along the Iodake coast.
When the hot water is mixed with seawater, dissolved alumina and silica are exsolved as white precipitates discoloring seawater (Photo shows Akayu Hot Spring on the left and Higashi Hot Spring in the center).
The sulfate acidic hot spring is formed when acidic fluid formed by mixing of volcanic gas and groundwater reacts with volcanic rocks to dissolve some components of the rocks.
Springs with dissolved iron-bearing carbonic acid
On the coast of Inamuradake (See Distribution map of volcanic gas and hot springs) are weakly acidic iron-bearing carbonate salt springs (Nagahama Hot Spring and Akayu Hot Spring) are distributed. There, precipitates of iron hydroxides are formed resulting in discolored sea water. At Iojima Port is Nagahama Hot Spring and seawater within the port is discolored by reddish brown precipitates of iron.
Hot brine spring
Seawater is heated to form hot brine springs at Sakamoto Hot Spring and Showa-iojima (See Distribution map of volcanic gas and hot springs).
Gas from the soil
In wide low ground within the Iojima, volcanic CO2 is discharged as soil gas (See Distribution map of volcanic gas and hot springs).
Volcanic CO2 gas discharge is particularly abundant around Iodake, caldera edge, and where hot springs are, and where the gas is abundantly discharged ground temperature tends to be high (Photo: Soil gas discharge zone). Volcanic CO2 gas in the soil is interpreted to correspond to the remaining gas after acid water-soluble components are removed during cooling and reaction with groundwater while rising from the depths.
Separated gas on the seafloor
Around the Higashi Hot Spring and the Showa-iojima (See Distribution map of volcanic gas and hot springs), separated gas bubbles are seen rising from the sea floor (Photo: Separated gas on the seafloor).
Similar with soil gases, seafloor gas consists of remaining components of volcanic gases after dissolving into seawater.
(Hiroshi SHINOHARA)