Kusatsu-Shirane Volcano
Introduction / Geology of Kusatsu-Shirane Volcano and environs / Geology of Kusatsu-Shirane Volcano
Eruptions of historical times
Rocks of Kusatsu-Shirane Volcano
Geochemistry of Kusatsu-Shirane Volcano
Compositions of volcanic gasses and their variation
Prediction of eruption, mitigation of hazard,
and benefit of volcano
Acknowledgment / References / Postscript
![]() PREV
PREV ![]() NEXT
NEXT
Geochemistry of Kusatsu-Shirane Volcano Compositions of volcanic gasses and their variation
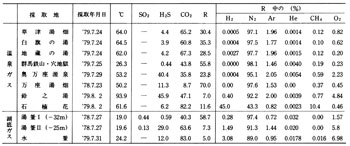
Many fumaroles exists on flanks and foot of Kusatsu-Shirane Volcano despite no lava has been erupted in recent years. Most fumaroles are concentrated in Sesshogawara, north of the summit, near Mizugama, Karabuki, and Jinetsu areas. The gas temperatures are comparatively low at 93 to 102?C as shown in ![]() Table 2. Except for water ( steam ), major components are H2S and CO2 with little R gas ( residual gas ). At times little SO2 and HCl are detected. Especially at Karabuki fumarole H2S is rich and at one time it reached 90 % ( excepting water ). The compositions of fumarolic gasses varies irregularly and at times increase of SO2 was detected.
Table 2. Except for water ( steam ), major components are H2S and CO2 with little R gas ( residual gas ). At times little SO2 and HCl are detected. Especially at Karabuki fumarole H2S is rich and at one time it reached 90 % ( excepting water ). The compositions of fumarolic gasses varies irregularly and at times increase of SO2 was detected.
Gasses erupt accompanying hot water spring and from lake bottoms. Compositions of some of them are shown in ![]() Table 3. The characteristic features of gas compositions are shown in
Table 3. The characteristic features of gas compositions are shown in ![]() Fig. 5.
Fig. 5.
Characteristics of hot and cold spring water
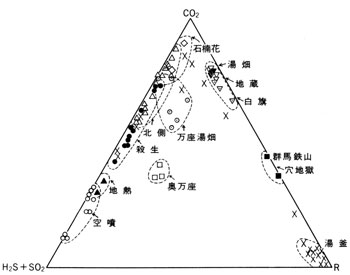
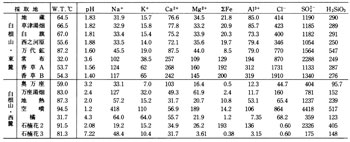
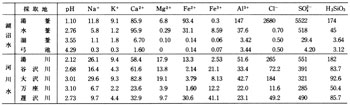
Many hot springs are distributed in this area and major ones are listed in Table 4. Kusatsu, Bandai, and Kagusa hot springs on the east foot of Kusatsu-Shirane Volcano are strongly acidic and although their pHs are different, overall compositions show similarity ( A in ![]() Fig.6 ). Those springs are understood to have common source at Fukigahara Pond on the flank of Kusatsu-Shirane Volcano according to previous researchers. The water flowed from there and through Oshi pyroclastic flow deposit under Aoba and Sessho Lavas passing fumarolic area at Sesshogawara reacting with volcanic gasses to become acid and dissolving surrounding rock compositions. Finally it discharges at Kusatsu Township and elsewhere as hot, strongly acid, and highly enriched in various compositions after mixing with other low temperature low concentration water such as water in Yazawahara pyroclastic flow.
Fig.6 ). Those springs are understood to have common source at Fukigahara Pond on the flank of Kusatsu-Shirane Volcano according to previous researchers. The water flowed from there and through Oshi pyroclastic flow deposit under Aoba and Sessho Lavas passing fumarolic area at Sesshogawara reacting with volcanic gasses to become acid and dissolving surrounding rock compositions. Finally it discharges at Kusatsu Township and elsewhere as hot, strongly acid, and highly enriched in various compositions after mixing with other low temperature low concentration water such as water in Yazawahara pyroclastic flow.
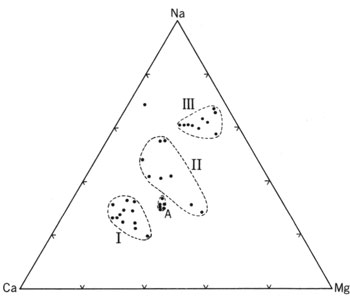
On the other hand many hot springs exist in the western foot area of the volcano such as Manza, Tachibana, and Shakunage, but the compositions of the water are variable as shown in ![]() Fig. 6, I to III. Those areas are covered by several lavas older than those in Kusatsu-Shirane Volcano and criss-crossed with faults. Thus the geology is rather complex and hot springs gush out through complex mechanism. The water perhaps represents different mixing ratio of I and III in
Fig. 6, I to III. Those areas are covered by several lavas older than those in Kusatsu-Shirane Volcano and criss-crossed with faults. Thus the geology is rather complex and hot springs gush out through complex mechanism. The water perhaps represents different mixing ratio of I and III in ![]() Fig. 6 in addition to dilution of those with surface water.
Fig. 6 in addition to dilution of those with surface water.
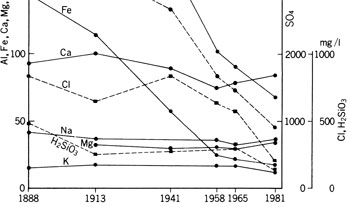
Among many hot springs, that of Kusatsu is best known and has been studied; for a long time. Compositional changes with time for the last 90 years since 1888 are shown in ![]() Fig. 7.
Fig. 7.
Water quality of lakes and rivers
Many lakes and pools of water accumulated in explosion craters exist in this area. Water quality for some of them is shown in ![]() Table 5. Among them Yugama on the summit of Kusatsu-Shirane Volcano is strongly acid with high concentrations of dissolved components. On the bottom of this lake is accumulated melted sulfur at 116?C carried up from depth by gas.
Table 5. Among them Yugama on the summit of Kusatsu-Shirane Volcano is strongly acid with high concentrations of dissolved components. On the bottom of this lake is accumulated melted sulfur at 116?C carried up from depth by gas.
Many streams flowing out from this area are strongly acid because of above mentioned acid hot springs and fumaroles besides wastewater from abandoned sulfur mines ( ![]() Table 5 ). Water of Yukawa and Yazawagawa Rivers shows strong influence of spring water, that of Osawagawa and Osozawagawa Rivers from abandoned sulfur mines, and that of Manzagawa River from both spring and mine waste water.
Table 5 ). Water of Yukawa and Yazawagawa Rivers shows strong influence of spring water, that of Osawagawa and Osozawagawa Rivers from abandoned sulfur mines, and that of Manzagawa River from both spring and mine waste water.
Mizugama eruption in 1976 and changes in volcanic gas and water compositions
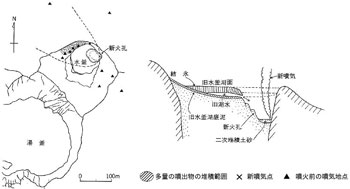
In March 1976 ( 51st year of Showa ) from the NE corner of frozen Mizugama crater lake a phreatic eruption occurred ( ![]() Fig. 8 ). It was 34th year since the last eruption in 1942 ( 17th year of Showa ) in Kusatsu-Shirane area. Also it was the first time in history for Mizugama to have erupted. Strong eruption was only once and because of the season ( winter ) there was no casualty. However, it caused changes in compositions of volcanic gas and water quality.
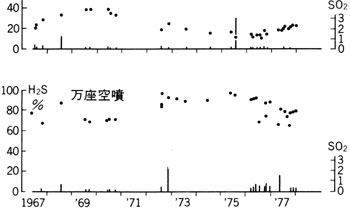
1 ) Since this activity a little SO2 has been detected in fumaroles of this area (
Fig. 8 ). It was 34th year since the last eruption in 1942 ( 17th year of Showa ) in Kusatsu-Shirane area. Also it was the first time in history for Mizugama to have erupted. Strong eruption was only once and because of the season ( winter ) there was no casualty. However, it caused changes in compositions of volcanic gas and water quality.
1 ) Since this activity a little SO2 has been detected in fumaroles of this area ( ![]() Fig. 4 ).
2 ) Within certain distance of fumaroles concentration in the atmosphere of H2S increased and the residence area of this gas increased.
3 ) Many new fumaroles with SO2 have been found near Mizugama.
4 ) Since a year before the eruption the water of Mizugama was suddenly depleted of Fe3+ with corresponding increase of Fe2+.
Those unusual precursors have been noted over a year and been watched carefully. That geochemical changes could proceed before phreatic eruption was a new fact recognized for the first time.
Fig. 4 ).
2 ) Within certain distance of fumaroles concentration in the atmosphere of H2S increased and the residence area of this gas increased.
3 ) Many new fumaroles with SO2 have been found near Mizugama.
4 ) Since a year before the eruption the water of Mizugama was suddenly depleted of Fe3+ with corresponding increase of Fe2+.
Those unusual precursors have been noted over a year and been watched carefully. That geochemical changes could proceed before phreatic eruption was a new fact recognized for the first time.
Alterations of ejecta in recent years and secondary minerals contained in them
This volcano has been active with repeated phreatic explosions without any new magmatic materials. The ejecta are altered materials from pre-existing rocks containing various secondary minerals. As an example chemical composition of ejecta from Mizugama in March 1976 is shown in ![]() Table 1. The confirmed secondary minerals included in this are, montmorillonite, kaolinite, pyrophyllite, opal, alunite, gypsum, anhydrite, and sulfur.
Table 1. The confirmed secondary minerals included in this are, montmorillonite, kaolinite, pyrophyllite, opal, alunite, gypsum, anhydrite, and sulfur.
Hydrogen sulfide automatic surveillance and warning system
Many high as well as low temperature fumaroles are distributed in Kusatsu-Shirane area, and depending on their localities and wind directions volcanic gas may stay or concentrated. Poisonous gas such as H2S concentration may reach dangerous level. In recent years distribution of such gas is examined for all the areas and potential hazard areas from H2S are identified ( cf. geologic map ).
On the map, areas with H2S concentration probably reach over 100 to 1,000 ppm in the air if climatic conditions are bad ( 1 ), and those possibly reach 10 to 100 ppm depending on the conditions ( 2 ) are shown. Those areas are now fenced and clearly designated as off limits with notice boards. As long as one keeps out of those designated areas there would be little danger. However, as the poisonous gas has high density as compared to air, if there is no wind or in quiet night with prevailing down draft, especially when the temperature of the gas is low with higher density than normal, the gas may concentrate in low lying depressions. As the gas has no smell with no color it is not easy to detect. If there is a smell of H2S, it is recommended that covering nose and mouth with wet cloth and escape from that area without lowering the posture as soon as possible. If treated in fresh air the fatality may be avoided.
In Sesshogawara and Manza areas where many people visit, H2S automatic surveillance system with warning device was installed. Since 1977 ( 52nd year of Showa ), if H2S concentration reaches dangerous level, the warning system consisting of 11 sensors and 5 warning devices would be automatically triggered to prevent accident.
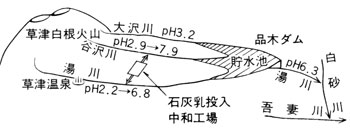
Neutralization of acid river water
As mentioned before, many rivers of this area are strongly acid and environmentally hazardous to downstream areas ( ![]() Table 5 ). Lime milk treatment ( neutralization ) of water of Yukawa River, the acidity of which is highest in the area, and to which most wastewater from Kusatsu Hot Spring is discharged, started in 1964 ( 39th year of Showa ) along with for water from Yazawagawa and Osawagawa Rivers running parallel to it. The improvement of water quality is clearly shown in
Table 5 ). Lime milk treatment ( neutralization ) of water of Yukawa River, the acidity of which is highest in the area, and to which most wastewater from Kusatsu Hot Spring is discharged, started in 1964 ( 39th year of Showa ) along with for water from Yazawagawa and Osawagawa Rivers running parallel to it. The improvement of water quality is clearly shown in ![]() Fig.9. Dam construction, power generation, bridge construction, irrigation, and fisheries are some of the fields most favorably affected in downstream rivers including Shirasuna and Azuma Rivers.
Fig.9. Dam construction, power generation, bridge construction, irrigation, and fisheries are some of the fields most favorably affected in downstream rivers including Shirasuna and Azuma Rivers.